Industrier
Forsikring
Industri og produksjon
Datacentre
Vindmøller og fornybar energi
Marine og offshore
Helsevesen
Utdanning
Mat og drikke
Forsikring
Vi bistår forsikringsselskap og takstmenn i små eller store skadesaker med å gjenopprette utstyr tilbake til sin opprinnelige tilstand, før skade, dermed sparer skadelidte tid og penger.
Industri og produksjon
Vi tilbyr vedlikehold og rensning av spesialutstyr. Vi har erfaring med industrimaskiner og har kunnskap om omkostningene og læringskurvene som er forbundet med nytt utstyr.
Datacentre
Vi gjenoppretter datasentre basert på de mest kostnadseffektive tiltakene, inkludert en analyse av gjenoppretting kontra erstatning.
Vindmøller og fornybar energi
Vårt team har håndtert hundrevis av prosjekter på et bredt spekter av merker og modeller, i over 60 land rundt om i verden, i mer enn 40 år.
Marine og offshore
Vi gjenoppretter datasentre basert på de mest kostnadseffektive tiltakene, inkludert en analyse av gjenoppretting kontra erstatning.
Helsevesen
Our engineers and technicians, many from the biomedical field, have provided guidance on recertification and reconditioning of medical equipment losses across the globe.
Utdanning
We work with all involved parties to mitigate loss after an incident as quickly as possible and get school back in session.
Mat og drikke
We restore Food and Beverage production equipment to pre-loss condition, ultimately saving clients time and money.

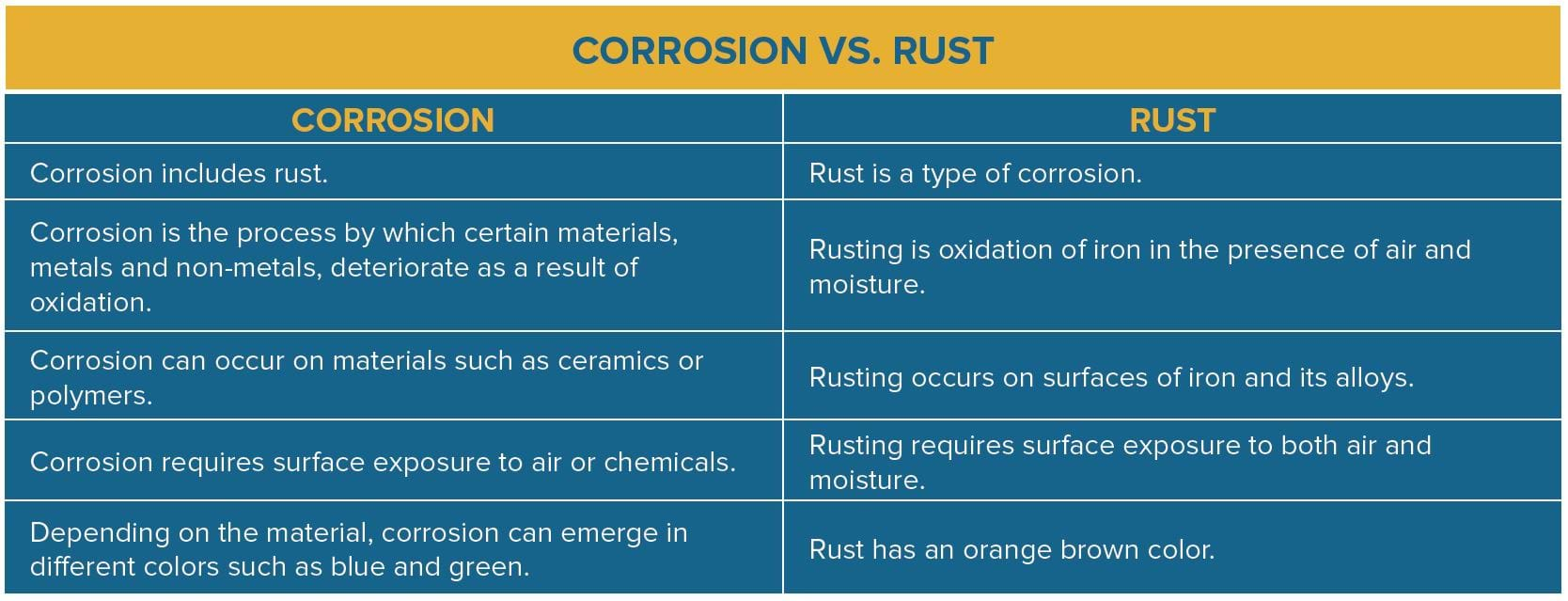 For more information on corrosion and rust, check out our whitepaper,
For more information on corrosion and rust, check out our whitepaper,